
The Full Story
conceptual design development of co2 conversion to methanol
This study’s main objective is to develop
alternative conceptual process line-ups for CO2 conversion to methanol.
For all processes, parametric studies, pinch analysis, heat integration and economic analysis are performed. Designs are developed in Aspen Plus simulation software.
executive summary
Two different alternatives are developed for methanol production;
-
CO2 is converted to methanol in a single reactor (CO2-based route)
-
CO2 is converted to CO in an RWGS reactor and
CO is converted to methanol in a separate reactor (CO-based route)
Sensitivity analyses are performed to find the optimum operating conditions for reactors.
1 Mt/yr (3 kt/d) methanol production is targeted for CO2-based route and
required fresh feeds are calculated accordingly.
The same fresh feeds are used in the CO-based route as well.
Heat integration is designed by using Aspen Energy Analyzer.
First, ISBL heat integrations are designed within the process streams.
Then cooling water, chilled water, and pressurized steam are utilized to supply the remaining energy requirements.
Capital (CAPEX) and operational (OPEX) costs are calculated for each route,
and results show that CO2-based route has less CAPEX and OPEX than CO-based route
but none of the processes is profitable at materials’ reference prices (as 2021).
Economic sensitivity analysis is performed for varying prices of raw materials and final products.
Studies show that each route can become profitable if the price(s) of raw materials,
methanol and hydrogen, decrease to certain levels.
Even though the CO-based route results in %10 more methanol production compared to CO2-based route,
the differences are minor and within the experimental error margin.
The main conclusion is that both alternatives for CO2 conversion methanol are more or less on par,
with small benefits for the CO2-based route.

methodology
In the development of any conceptual process design, methodology plays a vital role.
As the complexity of the process increases, the importance of methodology also increases exponentially.
The methodology applied in this project includes the following steps:
-
Ensure that the simulation software (Aspen Plus for this project) database contains all the chemicals involved in the design.
-
Select the physical and thermodynamic models that can represent the system best.
-
Acquire reaction kinetics for the reactions occurring in the reactors.
-
Integrate the reaction kinetics into the simulation software and validate the model with experimental results.
-
Define constraints, if applicable, such as the maximum pressure the reactor can maintain or the maximum temperature the catalyst can withstand.
-
Conduct parametric studies on reactor conditions to maximize productivity within the constraints.
-
Develop the reactor upstream process; i.e., prepare feeds from feed conditions to the reactor conditions.
-
Develop the reactor downstream process; i.e., separate the products at the reactor outlet in the desired way as ready-to-be-stored.
-
Connect the recycle streams, if applicable.
-
Conduct heat integration studies to increase plant efficiency and reduce operational expenses.
-
Conduct parametric studies to increase productivity or reduce costs.
-
Conduct economic analysis, determine OPEX and CAPEX, and construct tornado charts to visualize the impact of raw material and end product prices.
-
Report the findings.
No model can represent the reality with 100% accuracy. However, it is imperative to follow
a consistent methodology to ensure that the process is designed effectively, efficiently and closer to the reality.
This approach guarantees that the design is accurate and can produce the expected results.
By following this methodology, the project team can deliver a reliable and efficient process design
that meets the client's needs without having to spend excessive amount of time and money.
assumptions
-
CO2 and H2 are readily available at the site
at 25 degC and 1 bar.
-
Carbon capture from air and hydrogen electrolysis are out of this project’s scope.
-
The prices of CO2, H2 and methanol are taken as 0, 2000, 350 $ per ton, respectively.
-
One million tons per year of methanol production is aimed at least 99.85 weight % purity.
-
Debates regarding the carbon source and the reaction pathway towards methanol are still ongoing. A wide range of different kinetic models has been proposed with different underlying physical mechanisms.
-
Kinetic model is not explained here, rather available in the full report. Please contact us for more details.
reactions
Reactions involved in methanol synthesis are given below, as CO- and CO2- hydrogenation and RWGS reactions, respectively.
From a thermodynamic point of view,
methanol formation is favoured at low temperatures due to the exothermicity of reactions and high pressure due to the components’ stoichiometric ratio.
Reactions are highly exothermic overall which makes temperature control crucial.
Since reactions are well-known,
reaction kinetics are easy to acquire.

physical and thermodynamical models
Several factors need to be considered for selecting physical property method(s), and no single method can handle all systems.
In the selection process,
four factors should be considered;
-
the nature of the compounds
-
composition of the mixture
-
pressure and temperature range
-
availability of parameters
Four factors lead to two different models depending on the pressure level.
-
When pressure < 10 bar → NRTL-RK
-
When pressure > 10 bar → RKS with MHV-2

*Carlson, E., “Don’t Gamble with Physical Properties for Simulations”,
Chemical Engineering Progress, 1996.
reactor pressure
effect
Pressure is one of the crucial aspects of process flowsheet development.
While maintaining pressure to some level is a must for some processes,
further increase in pressure may increase the cost more than the benefits it brings.
It may also cause serious safety and operational problems.
Great care should be taken during the decision process of finding the optimum operating pressure point.
Methanol production from synthetic gas or CO2 requires at least 50 bar pressure to reach a satisfactory conversion level due to its reaction mechanism’s thermodynamic nature. However, since CO2 has a critical pressure of 72 bar, this sets an upper limit to our work because we want to compare the profitability and sustainability of CO- and CO2-based routes at the same operating conditions.
Pressure effect on conversions of pure CO2 (lower part) and CO-rich stream with 3% CO2 (upper part) on methanol synthesis
Since the stoichiometric numbers of compounds and reactants are equal for the RWGS reaction,
pressure has no significant effect. However, this is not the case for CO- and CO2-hydrogenation to methanol. Since increasing the pressure on gaseous reactions shifts the position of equilibrium towards
the side with fewer molecules, pressure increase results in shifting
both CO- and CO2- hydrogenation towards methanol’s side.
The CO- based stream yields 2-3 times more conversion than the CO2-based stream at each pressure level.
The CO-based stream is more sensitive to pressure change, whereas CO2 stream results in a more straight behavior.
Consequently, since higher pressure values enhance conversion towards methanol,
the highest possible pressure is aimed. However, the supercritical pressure of CO2 sets an upper limit at
72 bar. Hence, we set our operating pressure for CO- and CO2-based alternatives at 70 bar.
Although higher pressures are possible for CO-rich inlets,
we set both alternatives at the same pressure for the sake of fair comparison.

reactor temperature
effect
Temperature change has multiple competing effects. The most dominant effects are
the thermodynamic effect, which shifts equilibrium depending on the enthalpy of the reaction(s),
and the kinetic effect, which typically accelerates reaction rate(s) because temperature rise
increases the average kinetic energy of the reactant molecules.
On the other hand, temperature control has the utmost importance in a reactor to keep it productive enough and under control at the same time, to prevent the catalyst from any harm such as cooking and poisoning.
For the methanol synthesis, the CZA catalyst is used for both alternatives. Hence, care must be taken
to keep temperature below 250 degC to prevent/minimize hydrocarbon formation risk as a by-product.
The CZA catalyst is also known to be activated above approximately 200 degC.
Consequently, the possible operating temperature is relatively narrow,
but it can still make a difference at this high production level.
Figure in Pressure Effect section shows that increase in temperature enhances methanol production when CO2-rich stream is used as methanol synthesis reactor inlet. The reason for this behaviour is
the stable nature of CO2 molecules and low conversion values as a result. Naturally, 70 bar pressure level is not enough to make CO2 hydrogenation reaction approach its thermodynamic equilibrium in the reactor. Reactors are operated at around 5100 hr-1 GHSV, and required GHSV to reach thermodynamic equilibrium is around 150 hr-1 for CO2-rich stream according to our studies.
For CO2-rich stream, operating at higher temperature levels is always beneficial in production,
but catalyst should be protected from deactivation by keeping the temperature below/at 250 degC.
Kinetics vs thermodynamics effect with respect to temperature on CO-rich stream
CO-rich stream leads to higher conversion values compared to CO2-rich stream,
which is why CO-rich syngas is preferred for methanol synthesis in the industry. Because of this reason,
the methanol synthesis reactor operates closer to the thermodynamic equilibrium when
a CO-rich stream is used. The same study suggests that thermodynamic equilibrium
can be reached with approximately 4500 hr-1 GHSV.
Studies show that the increase in temperature enhances CO conversion when kinetics are dominant
However, after some point, thermodynamics start to dominate and
a further increase in temperature inhibits CO conversion.
As a result, for CO-rich stream, an optimum point at around 220 degC has found for operating temperature.

co2 content effect
CO2 content effect study is performed for the CO-based route to determine how much CO2 should be converted into CO in the RWGS reactor before sending the stream into the methanol synthesis reactor. CO2 addition into CO-rich inlet has a positive effect up to some point, but further addition inhibits the conversion. To find what content of CO2 gives the most conversion towards methanol, a parametric study is conducted with varying amount of CO2 mole fractions while introducing an SN-ratio and keep it equal to industry-standard 2.0 and overall streamflow unchanged.
In this parametric study, streams containing different amounts of CO2 (1%, 3%, 5%, 10% and 25% in molar base) while keeping the SN-ratio at 2.0 are sent to methanol synthesis reactor to investigate the CO2 content effect on methanol production. Results show that the most conversion value towards methanol is obtained when the molar composition of CO2 is around 3%, and decreases with less and further CO2 in the feed. Theoretically, our aim should be to convert as much CO2 in the RWGS reactor into CO, in such a way that around 3% CO2 would enter the methanol synthesis reactor. After CO2 content reaches 10%, the conversion decreases continuously, and the least conversion is obtained with pure CO2.

direct co2 conversion
process design
Pure CO2 and H2 are supplied as fresh feeds to the plant. How do pure CO2 and H2 are obtained is out of the scope of this project. Mixed-gases pass a series of compressors (C10x) and heat exchangers to get pressurized up to 71 bar, considering the pressure drop they would face before entering into the methanol reactor at 70 bar and 235 degC. CO2 conversion per pass in the isothermal multitubular reactor (R101) on
CZA catalyst is 21%, and this low conversion value leads to enormous unreacted CO2 and H2.
Simplified process flow diagram of direct CO2 route
Almost all of the methanol and water condense after the product stream’s temperature is decreased to
34 degC. Liquified products are separated from the unreacted gases in flash vessel (F101). Liquid product is directed into two distillation columns in series for end-product purification, and unreacted gases are recycled back with the fresh feed.
Unreacted gases from F101 are compressed up to 71 bar and recycled back into the reactor after 2.5% of it is purged to prevent accumulation. Water is separated from methanol and CO2 mixture in a distillation columng (DIST101). Lighter top stream is fed to DIST102 for methanol purification, in which methanol is purified
more than 99.95 wt-% purity. Unreacted gaseous stream is recycled back to mix with fresh feed.
Recycle stream consists of 89 wt-% CO2, 9 wt-% water, 1 wt-% H2 and trace amount of methanol.
The main advantage of this alternative is less equipment used in the design.
The energy requirement is also lower compared to the CO-based route.
The main disadvantage is the high amount of recycling ratios and bigger equipment requirements due to low conversion values obtained from stable CO2 molecules. Besides, a significant amount of CO2 and H2 are lost in the purge stream due to massive unreacted gas recycle from the top of F101.
However, these CO2 and H2 are not separated and sent back to the fresh feed
to have a fair comparison with the CO-based route.
Fresh feeds are calculated as 4462 t/d CO2 and 614 t/d H2.
Methanol production is 2947 t/d, water production is 1676 t/d, and 453 t/d (%2.5) of recycle is purged.

direct co2 conversion
heat integration
Heat exchangers within compressors in series are not included in the heat integration design
since they come with the compressors as a complete package. A temperature difference of nearly 170 degC between reactor feed and outlet suggests that the outlet stream can used to heat the feed.
Product stream and feed stream are sent into a tubular heat exchanger counter-currently at the heat exchanger (1), and streams exchange 54.2 MW of heat while feed stream and product stream leave the heat exchanger (1) at 139 and 159 degC, respectively. Since the feed stream needs to be heated to 235 degC, another heat exchanger is used for further heating in which pressurized steam (H101) is utilized.
Simplified representation of heat integration in direct CO2 route
134.2 MW of heat required for DIST101 reboiler.
Product stream enters the heat exchanger (2) counter-currently to provide 42.5 MW of this amount.
8.9 MW of remaining duty for DIST101 reboiler is supplied from H101 which heats the reactor feed,
and the remaining 82.6 MW of DIST101 reboiler duty is supplied from a pressurized steam.
The reactor outlet is still at 125 degC, which is used in the DIST102 reboiler in the heat exchanger (3).
Then it is cooled down to 34 degC by using cooling water and sent to flash vessel (F101).
Distillation column condensers are cooled down by chilled water.
Consequently, a total of 96.7 MW of heat integration is achieved.


direct co2 conversion
equipment design
Reactor
The design has only one reactor to convert CO2 and H2 into methanol.
Reactor type is selected as multitubular packed bed due to the following reasoning.
The reactions involved are highly exothermic which makes precise temperature control crucial.
Catalyst is a commercial heterogeneous CZA, and because stable CO2 leads to low conversion,
additional actions have to be taken. Increasing temperature above 250 degC would decrease
the methanol selectivity since methanol starts to convert into undesired dimethyl ether.
However, the temperature must be kept at the maximum possible to increase the conversion.
Space velocity is another parameter affecting the reactor and catalyst volume
Although less velocity would provide more time for CO2 molecules to react, 5000 1/hr is selected to reflect industry conditions and be able to provide 1 Mt per year methanol production.
Consequently, a multitubular packed bed reactor with 11-meter height, 24216 tubes with 6-cm diameter
is designed as 60% of the volume is filled with the CZA catalyst with
1775 kg/m3 density and 5.4 mm particle diameter.
Compressor
To pressurize fresh feeds and unreacted recycle stream, compressors are used in which
the pressure ratio across one stage is designed not to exceed 3.
Cooling water is used to cool down pressurized streams in inter-stage heat exchangers.
All compressors are operated by using electricity.
Compressors are designed to have 95% mechanical and 85% polytropic eciency.
Distillation column
Two distillation columns are operated in the design.
The number of stages, reflux ratio, reboiler-to-feed ratio, and feed stage are the design parameters.
Water is taken at more than 99.99% purity in DIST101,
whereas methanol is separated at 99.85% purity in DIST102.
Reboiler heat duties are supplied within the process as a result of heat integration.
DIST101 uses cooling water in its condenser, whereas DIST102 uses chilled water
since recycle stream has to cool down to 14 degC, and cooling water cannot meet this requirement.
Details of both distillation columns are given below.
Heat Exchangers
In heat exchanger design, minimum temperature approach of 5 degC is used when two sides are liquid,
10 degC when sides are liquid-gaseous, and 15-20 degC when two sides are gaseous.
For heat integration, first ISBL integration is performed within the process.
Then, OSBL is completed with cooling water, chilled water, 20- and 40-bar steam.
Heat exchanger design details are given below.


direct co2 conversion
economics, capex and opex
CAPEX and OPEX are calculated, and a sensitivity analysis is performed to see the effects of price fluctuations. For CAPEX, a calculation tool proposed in "Chemical Engineering Design Principles, Practice and Economics of Plant and Process Design, Towler, G. and R. Sinnott, 2013" is used to calculate the purchase cost of each equipment, and the price is adjusted from 2010 to 2019 by using CEPCI values.
Average CEPCI in 2010 and 2019 are taken as 532.9 and 607.5, respectively.
An installation factor is then used to calculate the installation cost for each equipment.
Purchase and installation of compressors, heat exchangers, a reactor, flash columns, and two distillation columns would cost 128.1 MM$ for direct CO2 to methanol route.
A detailed CAPEX breakdown is given below.
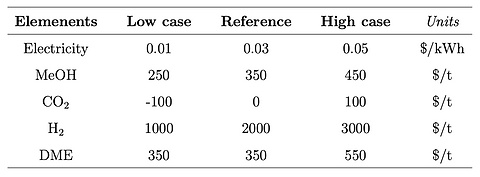
Sensitivity analysis is carried out with different scenarios in which prices of electricity, CO2, H2 and methanol are changed. A low-cost, a reference, and a high-cost scenario prices are determined for the four elements mentioned above. Prices for diffferent scenarios are given below.
Analysis are shown on tornado charts where red bars represent when the design becomes economically profitable in MM$ per year and yellow bars the scenarios when the design makes yearly losses in stated amounts. It is important to note that, when analyzing the affect of one parameter, the rest of the prices are kept constant at their reference prices. Wider bars means higher effects on OPEX.
Tornado chart shown below demonstrates that, when investigated one-by-one, H2 price has the most significant effect on OPEX, followed by CO2 and methanol. Electricity price has the least effect.
Effect of elements is also investigated through couples, i.e.,
CO2-methanol couple represents prices of both CO2 and methanol their lowest and highest together.
Not surprisingly, CO2 and H2 couple has the highest effect on OPEX. The effect of all couples is shown below.
Direct CO2 conversion to methanol is not economically profitable today, primarily due to the high price of pure H2. A 97 MM$ per year has to be invested in keeping the design (or such a plant) operational.
Detailed OPEX table for reference case can be seen below.
However, there are price scenarios where this route would become profitable as much as 256 MM$ per year.



co2 to co to methanol conversion
process design
Main difference of this route with direct CO2 route is that CO2 and H2 are first sent to
a reverse water gas shift (RWGS) reactor to convert CO2 to CO.
The reason for this addition is up to 2 times higher conversion values obtained in methanol synthesis reactor when the carbon source is CO, instead of CO2.
Simplified process flow diagram of CO2 to CO to methanol route (CO route)
CO2 and H2 pass through a series of compressors (C10x) before entering furnace (FR101) to get heated up to 700 degC and then sent to the RWGS reactor (R101). The outlet stream contains a significant amount of water due to the RWGS reaction. Therefore, this water is taken out in a flash vessel (F101) to
enhance CO conversion in the methanol reactor (R201). Since some methanol and CO2 go with the bottom stream of F101, it is beneficial to supply it to F202 to further separate water, methanol, and CO2.
Process stream taken from the top of F101 is sent to the methanol synthesis reactor (R201) with
70 wt-% H2, 21 wt-% CO and 9 wt-% CO2. Unreacted gases are separated in F201 and recycled back to the RWGS reactor after pressurized to 70 bar, since most of the unreacted carbon is CO2.
Liquified product stream from F201 is mixed with water from F101 and sent to F202.
A distillation column (DIST201) separates water at more than 99.99 wt-%, and another distillation column (DIST202) purifies methanol at more than 99.99 wt-%. Unreacted CO2 is recycled back to the fresh feed.
An important decision for this design is the operating temperature of the RWGS reactor
since it determines how much CO2 is converted into CO. CO2 content of the feed is crucial for
the methanol synthesis reactor since it can accelerate or inhibit methanol production.
Our studies suggest that operating an RWGS reactor at around 700 degC would be the most optimum point since a further increase in temperature would not enhance methanol production significantly
but increase operational costs. The reason is that although single-pass conversion increases with
increasing temperature, and hence CO content of the feed, the total mass going into the methanol synthesis reactor decreases. Water formation in the RWGS reaction is the reason for this total mass loss.
Consequently, an increase in CO conversion is balanced with mass loss to water,
and further temperature increase would not enhance end-methanol production.
Fresh feeds are kept equal with direct CO2 conversion to methanol route for fair comparison;
4462 t/d CO2 and 614 t/d H2. Methanol production is 10% higher in CO-route, with 3228 t/d of production. Water production is also higher, 1813 t/d. The main difference for enhanced methanol production is the less amount of purge stream of CO-based route, 35 t/d, compared to 453 t/d in CO2- based route.
The lost carbon in CO2-based route is converted into methanol in the CO-based route
due to higher conversion values obtained in the methanol synthesis reactor.
Overall mass balance comparison is given below.
No further detail will be provided for CO-route as it would be double explained.
Same procedure is followed to perform heat integration, CAPEX and OPEX calculations,
price fluctuation analysis and equipment design.
Purchase and installation costs of CO-route is given below. Results show that CO-route would be 11-15% costly compared to CO2-based route, which is not a surprise since CO-route consists of more equipment.




conclusion
Consequently, two flowsheet alternatives compete for profitability for CO2 conversion to methanol.
The first alternative is direct CO2 conversion to methanol in a single reactor.
The second alternative is converting CO2 to CO and then produce methanol from CO in separate reactors.
The exact amount of fresh feeds of CO2 and H2 are used to compare
both alternatives’ performance and profitability in a better perspective.
Direct CO2 conversion requires less equipment, but since the single-pass conversion is lower,
equipment sizes are bigger. The purge stream is also much bigger in the direct CO2 conversion since the single-pass conversion is small and the recycling stream is enormous.
CO conversion with RWGS reactor addition requires more equipment, but methanol production is higher.
The main reason for more methanol production is the significant amount of carbon loss
in the purge stream for direct CO2 conversion.
Methanol synthesis reactors are operated at 70 bar with CZA catalyst,
and Slotboom kinetics are used for modelling the reactions.
4.4 kt/d CO2 and 0.6 kt/d of H2 are converted into
2.9 and 3.2 kt/d of methanol in CO2- and CO-based routes, respectively.
Heat integration is designed first within process streams, and then
utilities such as cooling water and hot steam are used to complete the remaining energy requirements.
Capital (CAPEX) and operational (OPEX) costs are calculated for each route as
128 MM$ and 209 MM$/yr for CO2-based route, 147 MM$ and 257 MM$/yr for CO-based route, respectively.
As of 2021, none of the processes is profitable at their prices today.
Economic sensitivity analysis is performed for varying prices of raw materials and final products in their low and high case scenarios and tornado plots are obtained. Our studies show that
there are certain price scenarios in which each of the studied processes become profitable.
Consequently, this study shows that CO2 conversion to methanol for both routes are technically feasible.
The price scenarios which makes designs profitable are highly likely to be seen in the near future.
If the price of H2 can be decreased to certain levels, i.e., around $1000 per ton,
producing methanol from CO2 would be a great way to handle CO2 problem.
Purge stream is not utilized for the sake of even comparison with CO-based route, but it can be separated, and CO2 and H2 can be utilized to enhance the performance of the CO2-based design.
If you want to take a closer look at the full report or interested in more details
please do not hesitate to contact us!